In Chemical Reactions Atoms Are
In chemical reactions, atoms rearrange to form one or more different substances. In a chemical change, the properties that give a substance its identity change. Chemical equations show that in chemical reactions, atoms rearrange, but no atoms are lost or gained. In chemical reactions, energy is either absorbed or released. The one or more substances produced by a chemical reaction are called the product. In chemical reactions, the components of the reactants—the elements involved and the number of atoms of each—are all present in the product (s). Similarly, there is nothing present in the products that are not present in the reactants. The one or more substances produced by a chemical reaction are called the product. In chemical reactions, the components of the reactants—the elements involved and the number of atoms of each—are all present in the product (s). Similarly, there is nothing. This reaction could also be represented pictorially: Notice that the number of atoms of carbon is the same on both sides of the arrow. There is one carbon atom on the reactant side and one carbon atom on the product side. The same is true for oxygen except that there are two oxygen atoms on each side (remember that the subscript of two in the. What Happens to Atoms During a Chemical Reaction? Atoms in a Chemical Reaction. Atoms consist of a nucleus and surrounding electrons. The electrons arrange themselves in. Atoms are most stable when their valence electron shells are full. Depending on the atom's atomic number.
Learning Objectives
By the end of this section, you will be able to:
- Distinguish between kinetic and potential energy, and between exergonic and endergonic chemical reactions
- Identify four forms of energy important in human functioning
- Describe the three basic types of chemical reactions
- Identify several factors influencing the rate of chemical reactions
One characteristic of a living organism is metabolism, which is the sum total of all of the chemical reactions that go on to maintain that organism’s health and life. The bonding processes you have learned thus far are anabolic chemical reactions; that is, they form larger molecules from smaller molecules or atoms. But recall that metabolism can proceed in another direction: in catabolic chemical reactions, bonds between components of larger molecules break, releasing smaller molecules or atoms. Both types of reaction involve exchanges not only of matter, but of energy.
Chemical reactions require a sufficient amount of energy to cause the matter to collide with enough precision and force that old chemical bonds can be broken and new ones formed. In general, kinetic energy is the form of energy powering any type of matter in motion. Imagine you are building a brick wall. The energy it takes to lift and place one brick atop another is kinetic energy—the energy matter possesses because of its motion. Once the wall is in place, it stores potential energy. Potential energy is the energy of position, or the energy matter possesses because of the positioning or structure of its components. If the brick wall collapses, the stored potential energy is released as kinetic energy as the bricks fall.
In the human body, potential energy is stored in the bonds between atoms and molecules. Chemical energy is the form of potential energy in which energy is stored in chemical bonds. When those bonds are formed, chemical energy is invested, and when they break, chemical energy is released. Notice that chemical energy, like all energy, is neither created nor destroyed; rather, it is converted from one form to another. When you eat an energy bar before heading out the door for a hike, the honey, nuts, and other foods the bar contains are broken down and rearranged by your body into molecules that your muscle cells convert to kinetic energy.
Chemical reactions that release more energy than they absorb are characterized as exergonic. The catabolism of the foods in your energy bar is an example. Some of the chemical energy stored in the bar is absorbed into molecules your body uses for fuel, but some of it is released—for example, as heat. In contrast, chemical reactions that absorb more energy than they release are endergonic. These reactions require energy input, and the resulting molecule stores not only the chemical energy in the original components, but also the energy that fueled the reaction. Because energy is neither created nor destroyed, where does the energy needed for endergonic reactions come from? In many cases, it comes from exergonic reactions.
You have already learned that chemical energy is absorbed, stored, and released by chemical bonds. In addition to chemical energy, mechanical, radiant, and electrical energy are important in human functioning.
- Mechanical energy, which is stored in physical systems such as machines, engines, or the human body, directly powers the movement of matter. When you lift a brick into place on a wall, your muscles provide the mechanical energy that moves the brick.
- Radiant energy is energy emitted and transmitted as waves rather than matter. These waves vary in length from long radio waves and microwaves to short gamma waves emitted from decaying atomic nuclei. The full spectrum of radiant energy is referred to as the electromagnetic spectrum. The body uses the ultraviolet energy of sunlight to convert a compound in skin cells to vitamin D, which is essential to human functioning. The human eye evolved to see the wavelengths that comprise the colors of the rainbow, from red to violet, so that range in the spectrum is called “visible light.”
- Electrical energy, supplied by electrolytes in cells and body fluids, contributes to the voltage changes that help transmit impulses in nerve and muscle cells.
All chemical reactions begin with a reactant, the general term for the one or more substances that enter into the reaction. Sodium and chloride ions, for example, are the reactants in the production of table salt. The one or more substances produced by a chemical reaction are called the product.
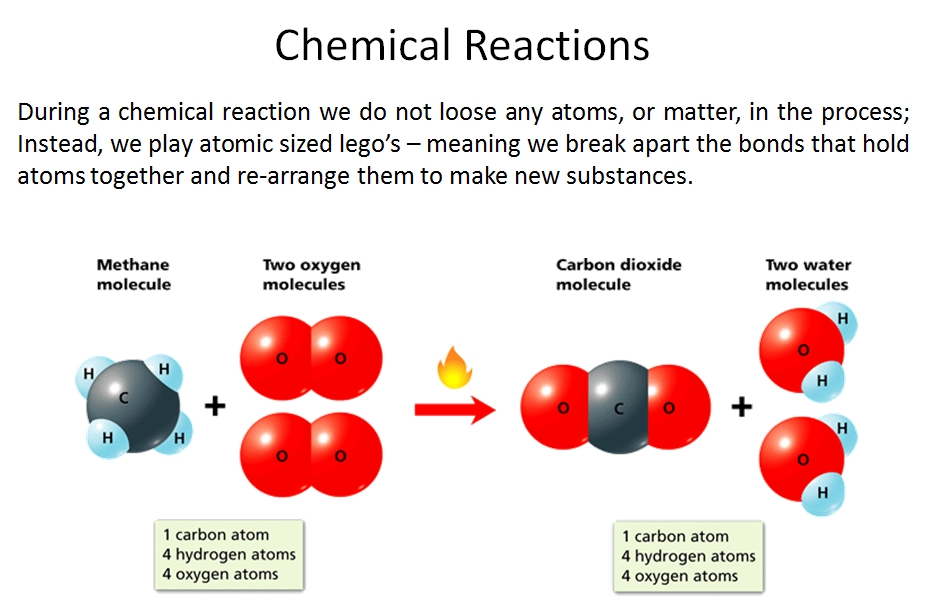
In chemical reactions, the components of the reactants—the elements involved and the number of atoms of each—are all present in the product(s). Similarly, there is nothing present in the products that are not present in the reactants. This is because chemical reactions are governed by the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Just as you can express mathematical calculations in equations such as 2 + 7 = 9, you can use chemical equations to show how reactants become products. As in math, chemical equations proceed from left to right, but instead of an equal sign, they employ an arrow or arrows indicating the direction in which the chemical reaction proceeds. For example, the chemical reaction in which one atom of nitrogen and three atoms of hydrogen produce ammonia would be written as . Correspondingly, the breakdown of ammonia into its components would be written as
Notice that, in the first example, a nitrogen (N) atom and three hydrogen (H) atoms bond to form a compound. This anabolic reaction requires energy, which is then stored within the compound’s bonds. Such reactions are referred to as synthesis reactions. A synthesis reaction is a chemical reaction that results in the synthesis (joining) of components that were formerly separate ([link]a). Again, nitrogen and hydrogen are reactants in a synthesis reaction that yields ammonia as the product. The general equation for a synthesis reaction is
In the second example, ammonia is catabolized into its smaller components, and the potential energy that had been stored in its bonds is released. Such reactions are referred to as decomposition reactions. A decomposition reaction is a chemical reaction that breaks down or “de-composes” something larger into its constituent parts (see [link]b). The general equation for a decomposition reaction is: .
An exchange reaction is a chemical reaction in which both synthesis and decomposition occur, chemical bonds are both formed and broken, and chemical energy is absorbed, stored, and released (see [link]c). The simplest form of an exchange reaction might be: . Notice that, to produce these products, B and C had to break apart in a decomposition reaction, whereas A and B had to bond in a synthesis reaction. A more complex exchange reaction might be:. Another example might be: .
In theory, any chemical reaction can proceed in either direction under the right conditions. Reactants may synthesize into a product that is later decomposed. Reversibility is also a quality of exchange reactions. For instance, could then reverse to . This reversibility of a chemical reaction is indicated with a double arrow: . Still, in the human body, many chemical reactions do proceed in a predictable direction, either one way or the other. You can think of this more predictable path as the path of least resistance because, typically, the alternate direction requires more energy.
If you pour vinegar into baking soda, the reaction is instantaneous; the concoction will bubble and fizz. But many chemical reactions take time. A variety of factors influence the rate of chemical reactions. This section, however, will consider only the most important in human functioning.
Properties of the Reactants
If chemical reactions are to occur quickly, the atoms in the reactants have to have easy access to one another. Thus, the greater the surface area of the reactants, the more readily they will interact. When you pop a cube of cheese into your mouth, you chew it before you swallow it. Among other things, chewing increases the surface area of the food so that digestive chemicals can more easily get at it. As a general rule, gases tend to react faster than liquids or solids, again because it takes energy to separate particles of a substance, and gases by definition already have space between their particles. Similarly, the larger the molecule, the greater the number of total bonds, so reactions involving smaller molecules, with fewer total bonds, would be expected to proceed faster.
In addition, recall that some elements are more reactive than others. Reactions that involve highly reactive elements like hydrogen proceed more quickly than reactions that involve less reactive elements. Reactions involving stable elements like helium are not likely to happen at all.
Temperature
Nearly all chemical reactions occur at a faster rate at higher temperatures. Recall that kinetic energy is the energy of matter in motion. The kinetic energy of subatomic particles increases in response to increases in thermal energy. The higher the temperature, the faster the particles move, and the more likely they are to come in contact and react.

Concentration and Pressure
If just a few people are dancing at a club, they are unlikely to step on each other’s toes. But as more and more people get up to dance—especially if the music is fast—collisions are likely to occur. It is the same with chemical reactions: the more particles present within a given space, the more likely those particles are to bump into one another. This means that chemists can speed up chemical reactions not only by increasing the concentration of particles—the number of particles in the space—but also by decreasing the volume of the space, which would correspondingly increase the pressure. If there were 100 dancers in that club, and the manager abruptly moved the party to a room half the size, the concentration of the dancers would double in the new space, and the likelihood of collisions would increase accordingly.
Enzymes and Other Catalysts
For two chemicals in nature to react with each other they first have to come into contact, and this occurs through random collisions. Because heat helps increase the kinetic energy of atoms, ions, and molecules, it promotes their collision. But in the body, extremely high heat—such as a very high fever—can damage body cells and be life-threatening. On the other hand, normal body temperature is not high enough to promote the chemical reactions that sustain life. That is where catalysts come in.
In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any change. You can think of a catalyst as a chemical change agent. They help increase the rate and force at which atoms, ions, and molecules collide, thereby increasing the probability that their valence shell electrons will interact.
The most important catalysts in the human body are enzymes. An enzyme is a catalyst composed of protein or ribonucleic acid (RNA), both of which will be discussed later in this chapter. Like all catalysts, enzymes work by lowering the level of energy that needs to be invested in a chemical reaction. A chemical reaction’s activation energy is the “threshold” level of energy needed to break the bonds in the reactants. Once those bonds are broken, new arrangements can form. Without an enzyme to act as a catalyst, a much larger investment of energy is needed to ignite a chemical reaction ([link]).
Enzymes are critical to the body’s healthy functioning. They assist, for example, with the breakdown of food and its conversion to energy. In fact, most of the chemical reactions in the body are facilitated by enzymes.
Chemical reactions, in which chemical bonds are broken and formed, require an initial investment of energy. Kinetic energy, the energy of matter in motion, fuels the collisions of atoms, ions, and molecules that are necessary if their old bonds are to break and new ones to form. All molecules store potential energy, which is released when their bonds are broken.
Four forms of energy essential to human functioning are: chemical energy, which is stored and released as chemical bonds are formed and broken; mechanical energy, which directly powers physical activity; radiant energy, emitted as waves such as in sunlight; and electrical energy, the power of moving electrons.
Chemical reactions begin with reactants and end with products. Synthesis reactions bond reactants together, a process that requires energy, whereas decomposition reactions break the bonds within a reactant and thereby release energy. In exchange reactions, bonds are both broken and formed, and energy is exchanged.
The rate at which chemical reactions occur is influenced by several properties of the reactants: temperature, concentration and pressure, and the presence or absence of a catalyst. An enzyme is a catalytic protein that speeds up chemical reactions in the human body.
The energy stored in a foot of snow on a steep roof is ________.
- potential energy
- kinetic energy
- radiant energy
- activation energy
The bonding of calcium, phosphorus, and other elements produces mineral crystals that are found in bone. This is an example of a(n) ________ reaction.
- catabolic
- synthesis
- decomposition
- exchange
is a general notation for a(n) ________ reaction.
- anabolic
- endergonic
- decomposition
- exchange
________ reactions release energy.
- Catabolic
- Exergonic
- Decomposition
- Catabolic, exergonic, and decomposition
Which of the following combinations of atoms is most likely to result in a chemical reaction?
- hydrogen and hydrogen
- hydrogen and helium
- helium and helium
- neon and helium
Chewing a bite of bread mixes it with saliva and facilitates its chemical breakdown. This is most likely due to the fact that ________.
- the inside of the mouth maintains a very high temperature
- chewing stores potential energy
- chewing facilitates synthesis reactions
- saliva contains enzymes
Is this a legitimate example of an exchange reaction? Why or why not?
It is not. An exchange reaction might be or . In all chemical reactions, including exchange reactions, the components of the reactants are identical to the components of the products. A component present among the reactants cannot disappear, nor can a component not present in the reactants suddenly appear in the products.
When you do a load of laundry, why do you not just drop a bar of soap into the washing machine? In other words, why is laundry detergent sold as a liquid or powder?
Recall that the greater the surface area of the reactants, the more quickly and easily they will interact. It takes energy to separate particles of a substance. Powder and liquid laundry detergents, with relatively more surface area per unit, can quickly dissolve into their reactive components when added to the water.
Glossary
- activation energy
- amount of energy greater than the energy contained in the reactants, which must be overcome for a reaction to proceed
- catalyst
- substance that increases the rate of a chemical reaction without itself being changed in the process
- chemical energy
- form of energy that is absorbed as chemical bonds form, stored as they are maintained, and released as they are broken
- concentration
- number of particles within a given space
- decomposition reaction
- type of catabolic reaction in which one or more bonds within a larger molecule are broken, resulting in the release of smaller molecules or atoms
- enzyme
- protein or RNA that catalyzes chemical reactions
- exchange reaction
- type of chemical reaction in which bonds are both formed and broken, resulting in the transfer of components
- kinetic energy
- energy that matter possesses because of its motion
- potential energy
- stored energy matter possesses because of the positioning or structure of its components
- product
- one or more substances produced by a chemical reaction
- reactant
- one or more substances that enter into the reaction

- synthesis reaction
- type of anabolic reaction in which two or more atoms or molecules bond, resulting in the formation of a larger molecule
Unit Summary
*REMOTE LEARNING GUIDE FOR THIS UNIT NOW AVAILABLE!*
CLICK DOWNLOAD TO ACCESS
Students' conceptual understanding of chemical reactions is foundational to much science learning. Understanding atomic level reactions is crucial for learning physical, life, earth, and space science. Even more importantly, they open up new windows of curiosity for students to see the world around them. By seventh grade, students are ready to take on the abstract nature of the interactions of atoms and molecules far too small to see.
To pique students’ curiosity and anchor the learning for the unit in the visible and concrete, students start with an experience of observing and analyzing a bath bomb as it fizzes and eventually disappears in the water. Their observations and questions about what is going on drive learning that digs into a series of related phenomena as students iterate and improve their models depicting what happens during chemical reactions. By the end of the unit, students have a firm grasp on how to model simple molecules, know what to look for to determine if chemical reactions have occurred, and apply their knowledge to chemical reactions to show how mass is conserved when atoms are rearranged.
Embedded in this unit are a variety of assessments, including self, peer, formative, and summative assessment tasks. This unit concludes with a transfer task in which students apply what they have figured out to two different related phenomena, elephant’s toothpaste and the crumbling of the marble that makes up the Taj Mahal.
Unit Examples
Additional Unit Information
This unit builds toward the following NGSS Performance Expectations (PEs):
- MS-PS1-1: Develop models to describe the atomic composition of simple molecules and extended structures. [Clarification Statement: Emphasis is on developing models of molecules that vary in complexity. Examples of simple molecules could include ammonia and methanol. Examples of extended structures could include sodium chloride or diamonds. Examples of molecular-level models could include drawings, 3D ball and stick structures, or computer representations showing different molecules with different types of atoms.] [Assessment Boundary: Assessment does not include valence electrons and bonding energy, discussing the ionic nature of subunits of complex structures, or a complete description of all individual atoms in a complex molecule or extended structure is not required.]
- MS-PS1-2: Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred. [Clarification Statement: Examples of reactions could include burning sugar or steel wool, fat reacting with sodium hydroxide, and mixing zinc with hydrogen chloride.] [Assessment boundary: Assessment is limited to analysis of the following properties: density, melting point, boiling point, solubility, flammability, and odor.]
- MS-PS1-5: Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved. [Clarification Statement: Emphasis is on law of conservation of matter and on physical models or drawings, including digital forms, that represent atoms.] [Assessment Boundary: Assessment does not include the use of atomic masses, balancing symbolic equations, or intermolecular forces.]
The following PE will be developed over three OpenSciEd units; OpenSciEd Unit 6.1: Why do we sometimes see different things when looking at the same object? (One-way Mirror Unit), OpenSciEd Unit 7.1: How can we make something new that was not there before? (Bath Bombs Unit), and OpenSciEd Unit 8.2: How can a sound make something move? (Sound Unit). This unit will address only the chemical inputs that transmit signals to the brain through smell. The other units will address electromagnetic and mechanical inputs, as well as the connection to signals processing in the brain, resulting in immediate behaviors or memories.
- MS-LS1-8. Gather and synthesize information that sensory receptors respond to stimuli by sending messages to the brain for immediate behavior or storage as memories.[Assessment boundary: Assessment does not include mechanisms for transmission of this information]
- MS-LS1.D: Information Processing: Each sense receptor responds to different inputs (electromagnetic, mechanical, chemical), transmitting them as signals that travel along nerve cells to the brain. The signals are then processed in the brain, resulting in immediate behaviors or memories.
PS1.A: Structure and Properties of Matter
- Substances are made from different types of atoms, which combine with one another in various ways.
- Atoms form molecules that range in size from two to thousands of atoms.
- Solids may be formed from molecules, or they may be extended structures with repeating subunits (e.g., crystals).
- Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it.
PS1.B: Chemical Reactions
- Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants.
- The total number of each type of atom is conserved, and thus the mass does not change.
In Chemical Reactions Atoms Are Quizlet
LS1-D: Information Processing
- Each sense receptor responds to different inputs (electromagnetic, mechanical, chemical), transmitting them as signals that travel along nerve cells to the brain. The signals are then processed in the brain, resulting in immediate behaviors or memories.
Disciplinary Core Ideas are reproduced verbatim from A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas. DOI: https://doi.org/10.17226/13165. National Research Council; Division of Behavioral and Social Sciences and Education; Board on Science Education; Committee on a Conceptual Framework for New K-12 Science Education Standards. National Academies Press, Washington, DC. This material may be reproduced and used by other parties with this attribution. If the original material is altered in any way, the attribution must state that the material is adapted from the original.
While this unit engages students in multiple SEPs across the lesson level performance expectations for all the lessons in the unit, there are three focal practices that this unit targets to support students’ development in a learning progression across the 7th grade year for the SEPs. These are:
- Constructing Explanations and Designing Solutions
- Analyzing and Interpreting Data
- Engaging in Argument from Evidence
In addition, there are two supporting practices that students will utilize over the course of the unit. These practices are two that students have developed over the course of 6th grade in the OpenSciEd sequence and will be used as supporting practices in this unit:

- Developing and Using Models
- Planning and Carrying Out Investigations
While this unit engages students in multiple CCCs across the lesson level performance expectations for all the lessons in the unit, there are three focal practices that this unit targets to help support students’ development in a learning progression. These are:
- Patterns
- Scale, Proportion, and Quantity
- Energy and Matter
The bolded sections of the related common core math standards are ones that students engage in.
In Chemical Reactions Atoms Are
Density is a property that students measure, graph, and calculate from mass and volume data in Lesson 8. They will be using the following two math concepts in that lesson:
- CCSS.MATH.CONTENT.7.RP.A.2.A Decide whether two quantities are in a proportional relationship, e.g., by testing for equivalent ratios in a table or graphing on a coordinate plane and observing whether the graph is a straight line through the origin.
- CCSS.MATH.CONTENT.7.RP.A.2.B Identify the constant of proportionality (unit rate) in tables, graphs, equations, diagrams, and verbal descriptions of proportional relationships.
Because this unit is taught using a conceptual approach to developing a model of matter that requires the existence of compound particles and smaller constituent parts (atoms), pre-teaching the idea that atoms exist and that they make up molecules is counterproductive to the trajectory of this unit. Students may have heard of the words “atoms” and “molecules” in other contexts and should be encouraged to try to apply any ideas about the particulate nature of matter they may bring to the table in the first part of the unit. But, since OpenSciEd units in 6th grade develop a particulate model of matter that doesn’t distinguish between molecules and atoms, the middle of this unit will be the first time that students will find the need for such distinction based on something they can’t explain about the anchoring phenomena. Many subsequent units in 7th grade OpenSciEd will use the ideas developed in this unit, to explain other phenomena, and will rely on the development of the following ideas developed in this unit. The unit that requires each idea listed here is identified in parentheses.
In Chemical Reactions Atoms Are Combined Separated Or Rearranged
- Every substance has characteristic properties that can be used to identify it (e.g., solubility, odor, melting point, boiling point, flammability, density, color). These do not change regardless of the amount of the substance. (7.2, 7.3)
- Substances are made from different types of atoms, which combine with one another in various ways. The number, type, and arrangement of atoms in the molecules that make up a substance are unique to that substance. (7.2)
- Atoms form molecules. (7.2, 7.3, 7.4)
- In a chemical reaction, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants. (7.2, 7.3, 7.4)
- In a chemical reaction, the total number of each type of atom is conserved, and thus the mass does not change. (7.2, 7.3, 7.4)
- There are two ways to break apart matter—physical processes and chemical processes. (7.3, 7.4)
- Chemical processes involve the rearrangement of particles that make up the matter; this includes chemical reactions, phase changes, and dissolving. (7.2)
Chemical Reaction Examples
- Dawn Novak, Unit Lead, BSCS Science Learning
- Michael Novak, Field Test Unit Lead, Northwestern University
- Holly Hereau, Writer, BSCS Science Learning
- Gail Housman, Writer, Northwestern University
- Betty Stennett, Writer, BSCS Science Learning
- Keetra Tipton, Writer, Sunset Ridge School, Northfield, IL
- Wayne Wright, Writer, BSCS Science Learning
- Renee Affolter, Reviewer, Boston College
- Tyler Scaletta, Pilot Teacher, Alcott College Prep Elementary School, Chicago Public Schools
- Katie Van Horne, Assessment Specialist
- Joseph Krajcik, Unit Advisory Chair, Michigan State University
- Michael Clinchot, Teacher Advisor, John D. O’Bryant School of Mathematics and Science
- Brian MacNevin, Teacher Advisor, Northwest Educational Service District 189
BSCS Science Learning
Chemical Reactions Unit Test Quizlet
- Christine Osborne, Copyeditor, Independent Contractor
- Denise Rubens, Copyeditor, Independent Contractor
- Stacey Luce, Editorial Production Lead
- Valerie Maltese, Marketing Specialist & Project Coordinator
- Renee DeVaul, Project Coordinator and Copyediting
- Chris Moraine, Multimedia Graphic Designer
In Chemical Reaction Atoms Are Brainly
An integral component of OpenSciEd’s development process is external validation of alignment to the Next Generation Science Standards by NextGenScience’s Science Peer Review Panel using the EQuIP Rubric for Science. We are proud that this unit has earned the highest score available and has been awarded the NGSS Design Badge. You can find additional information about the EQuIP rubric and the peer review process at the nextgenscience.org website.
